Boiling Vaporization Melting and Condensation Are Described Best as
All phase changes are accompanied by changes in the energy of a system. The substance changes from a.
Not change its melting point b.
. The substance changes from a gas to a liquid. The point at which liquid changes to vapor D. When normal atmospheric conditions are present water is constantly being cycled between liquid and gaseous states by which of the following pairs of processes.
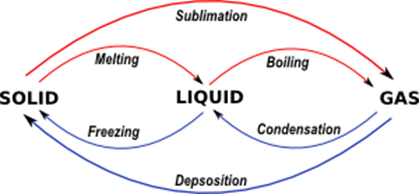
Fe s Fe t O vaporization condensation sublimation freezing 0 0 0 0 deposition melting. Melting- The process by which a substance changes from its solid state to its liquid state. Boiling happens throughout the liquid.
The reversed process of melting is a. Select all the statements that correctly describe heat of vaporization. Select the name of the physical state transition that is described in the following process.
Molecules would speed and spread apart. Learn vocabulary terms and more with flashcards. Evaporation- A process by which a substance changes from its liquid state to its gas state by random particle movement.
The atoms of a. Fusion vaporization and sublimation are endothermic processes whereas freezing condensation and deposition are exothermic processes. Boiling point is when liquid turns into vapor.
The temperature of vaporization aka boiling point is the same as the temperature of condensation where vapor changes to liquid. Simply put boiling is the process by which a liquid becomes a gas while condensation is the opposite. If a substance expands on melting increased pressure acting on it will a.
A water has a low heat of vaporization because it has weak intermolecular forces b heat of vaporization is the amount of energy needed to vaporize 1 g of a substance c the amount of energy released in condensation equals the amount of energy absorbed during vaporization. Freezing and condensation boiling and deposition condensation and melting vaporization and sublimation. When the liquid is heated and attains the boiling point the vaporization process is called boiling.
The boiling point of water in a pressure cooker is raised by. Which is NOT an endothermic process. The point at which liquid changes to solid B.
Melting point and condensation C. Turgor pressure and filtration B. However the heat of vaporization is something else.
Similarly the temperature of melting is the same as the temperature of freezing. The diagram shows changes of state between solid liquid and gas. Freezing- The process by which a substance changes from its liquid state into its solid state.
The point at which solid changes to liquid. Boiling happens throughout the liquid. Condensation is the opposite since it is the turning point.
Identify the different transition points between the phases of matter vaporization melting boiling freezing condensation sublimation Melting. Select the single best answer. Molecules will slow down and get closer together.
Changes of state are examples of phase changes or phase transitions. Which statement best describes the energy changes associated with evaporation and boiling. Start studying Solid Liquid Gas Freezing Melting Evaporation Boiling Vaporization Condensation Sublimation.
This is because the heating process convection heats up the entire liquid. It is the process by which a gas becomes a liquid. The correct answer is the first option.
Chemistry questions and answers. Increase its melting point c. Heat is when thermal energy is transferred and temperature measures these two things.
The temperature at which a substance changes from a solid to a. The point of condensation is the best description if the heating curve is reversed. The substance changes back from the solid to the liquid.
Deposition melting vaporization sublimation. Decrease its melting point d. Which option best describes the term boilingA.
The point at which vapor changes to gas or liquidC. The change in state from a solid to a liquid.

What Is Vaporization Geeksforgeeks

Change Of State Of A Matter Melting Fusing Evaporation Condensation

Art The Phase Changes Of Matter Include Melting Freezing Evaporation Condensation Deposition And Sublima Matter Science States Of Matter Changes In Matter
No comments for "Boiling Vaporization Melting and Condensation Are Described Best as"
Post a Comment